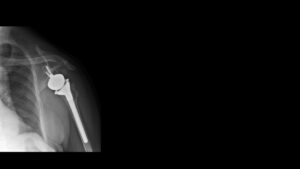
Exploring Treatment Options for Exactech Shoulder Issues: Surgery vs. Non-Surgical Approaches
The U.S. Food and Drug Administration (FDA) has issued a Safety Communication advising healthcare providers to stop implanting Equinoxe Shoulder System joint replacement devices manufactured by Exactech. The Safety Communication, which the FDA published on January 16, 2024, identifies issues with Exactech’s packaging that can cause oxidation leading to premature device degradation and failure.
In its Safety Communication, the FDA also includes an advisory for patients. While it does not recommend revision surgery to remove “well-functioning” Equinoxe Shoulder System implants, the FDA warns that “new or worsening pain or swelling, inability to use your arm, grinding or other noise, or weakness around your implanted device” may all be signs that revision surgery is necessary.
Revision Surgery for Compromised Equinoxe Shoulder System Implants
Once an Equinoxe Shoulder System joint replacement device starts to crack or deteriorate, there is no chance of the device repairing itself. In fact, for many patients, the problem will only continue to get worse. As a result, when a defective shoulder implant starts to negatively affect a patient’s health, revision surgery may be the only long-term solution.
While revision surgery may be necessary in many cases, this procedure itself presents risks. All surgeries do. This includes risks of complications during surgery as well as risks associated with the new shoulder replacement device. For example, the Mayo Clinic warns of risks including:
- Blood clots
- Dislocation
- Fracture
- Implant loosening
- Infection
- Nerve damage
- Rotator cuff failure
However, these risks may not be present in all cases, and, for many patients, the risks of keeping their defective Exactech shoulder replacement device will far outweigh the risks of having it removed and replaced. Ultimately, whether surgery is the best option for any patient will depend on his or her individual circumstances—and all patients should make their decisions in close consultation with their healthcare providers.
When weighing the benefits and risks of revision surgery, it is also important to consider the timeline and likely outcomes of the recovery and rehabilitation processes. These can vary greatly depending on the nature and severity of the damage caused by a defective Equinoxe Shoulder System joint replacement device. In a best-case scenario, recovery and rehabilitation can take about 12 weeks, although, for patients who have suffered significant harm from their Exactech shoulder implants, these processes could take significantly longer. Permanent complications from the defect may be a concern as well—and when this is the case, patients will want to take this into account when weighing the risks and rewards of revision surgery.
Alternative Treatment Options When Surgery Isn’t Necessary
While revision surgery will be necessary in many cases, some patients will have other options available. As the FDA explains in its Safety Communication regarding Exactech’s defective shoulder replacement devices, when talking to patients about surgery, healthcare providers should “[a]s a part of shared decision-making, discuss the benefits and risks of all relevant treatment options with your patients.”
What are the potential alternatives to surgery? Recognizing that individual patients’ circumstances will vary, some examples of possible options include:
- Corticosteroid injections
- Massage therapy
- Pain management
- Physical and occupational therapy
- Stem cell therapy
When considering these as alternatives to possible revision surgery to replace a defective Equinoxe Shoulder System implant, it is important for patients and their doctors to keep in mind the unique risks associated with these defective devices. While the alternatives listed above can often be good options for patients who are considering shoulder replacement for the first time, the need to remove a degrading or cracked shoulder implant may ultimately outweigh the benefits of pursuing a less-invasive alternative. Your doctor should be able to help you decide which option is best for you.
Covering the Costs of Surgery or Other Treatment for a Defective Equinoxe Shoulder System Implant
Regardless of which option is best for you, dealing with the effects of a defective Equinoxe Shoulder System implant can be costly. For most patients, there are both financial and non-financial costs involved. Examples of the financial costs associated with recovering from Exactech shoulder issues include:
- Medical Bills – This includes the cost of diagnosis, consultation, surgery, alternate treatment, and/or therapy and rehabilitation.
- Other Medical Expenses – This includes the cost of corticosteroids, pain medications, surgical wound care, and/or other necessary medications or medical supplies.
- Loss of Income – Individuals who are forced to deal with the effects of defective Exactech shoulder implants will often miss a significant amount of time from work during the recovery process. They may face long-term (or permanent) work restrictions due to complications as well.
Along with these financial costs, the stress and complications caused by defective Equinoxe Shoulder System implants can also lead to non-financial costs such as pain and suffering, loss of consortium and companionship, and loss of enjoyment of life.
For patients affected by defective Equinoxe Shoulder System replacement devices, covering these costs may involve filing a lawsuit against Exactech. Companies can (and should) be held fully responsible when they sell defective medical devices. Lawyers at Searcy Denney are actively pursuing claims against Exactech on behalf of patients who suffered harm caused by defective Equinoxe Shoulder System implants, and we have successfully pursued claims against the company related to its defective knee and hip implants in the past.
Finding out if you have a claim starts with scheduling a free, no-obligation consultation. During this consultation, an Exactech lawyer at Searcy Denney will assess your individual circumstances to determine if you have grounds to file a lawsuit. If you do, your lawyer will also provide a preliminary assessment of how much you may be able to recover, and then you can use this information to make smart decisions about your next steps.
Do You Have a Claim Against Exactech? Find Out for Free
Do you need to know more about filing a lawsuit against Exactech related to a defective Equinoxe Shoulder System implant? If so, we invite you to get in touch. To speak with an experienced Exactech lawyer at Searcy Denney in confidence, please call 800-780-8607 or request a free consultation online today.
Share This